Density temperature relationship formula for liquid
Therefore the change in temperature is Delta T 154circ C How to Calculate the Amount of. H_v fracqm Where.
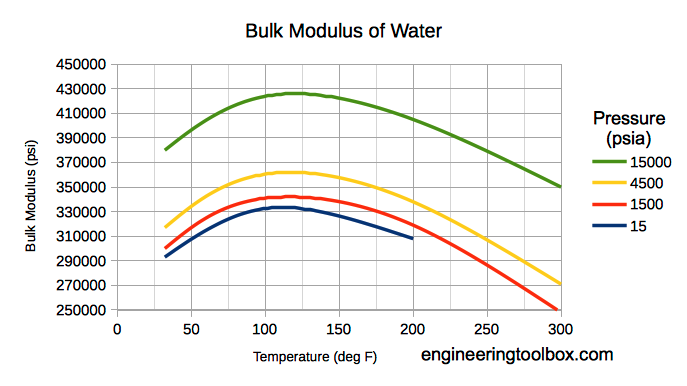
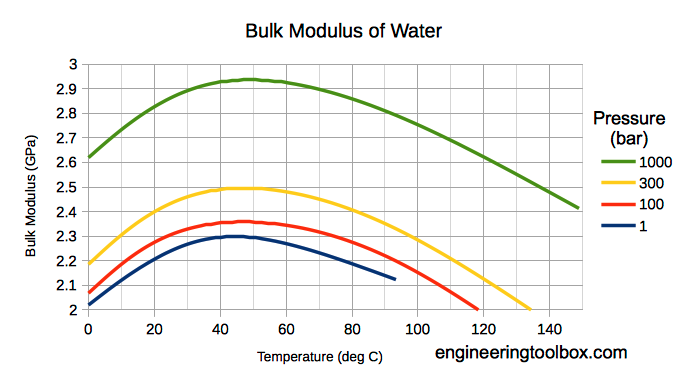
Liquids Densities Vs Pressure And Temperature Change
Density equals the number of molecules and molecular weight per volume occupied while viscosity is a measurement of the intermolecular forces between the molecules in a gasliquidfluid.
. It also changes with variation in atmospheric pressure temperature and humidityAt 101325 kPa abs and 20 C 68 F air has a density of approximately 1204 kgm 3 00752 lbcu ft according to the. Delta T fracQmc This is sometimes referred to as the change in temperature formula. Pressure cooking is inherently safer than normal cooking at ANY pressure.
The accepted density of this liquid is 0789 gcm 3 and its energy density is 268 mega-joules per kg. A liquids temperature will affect its vapor pressure. Both density and viscosity decrease with the increase in temperature.
In some cases for instance in the. This is because it is cooking at higher than boiling point for any given altitude. The heat of vaporization formula can be written as based on entropy and enthalpy of vaporization as well as their relationship.
Our air density calculator uses the following formula. For a given time more bugs will be killed. When it is cooled from room temperature the liquid water tends to become increasingly dense similar to.
However viscosity mostly has an exponential relationship with temperature. The formula you cite proves the relationship is linear. It says for a given volume Pressure is proportional to temperature.
The virtual temperature T v may used in the following formula to calculate the density altitude. It is the temperature at which the water vapor contained in air reaches its saturation state. Temperature T 5 C.
B Bulk modulus of the liquid. 53 g 1 mol7811 g 0679 mol. It compares the density of the liquid to the density of water so the result is in the form of a ratio or percentage.
ρ Density of the liquid. As we know that specific heat ratio in. The formula used for calculating vapor pressure given a change in the vapor pressure over time is known as.
If the density of the object is more than the density of the liquid the object will sink in the liquid. The density of air or atmospheric density denoted ρ is the mass per unit volume of Earths atmosphereAir density like air pressure decreases with increasing altitude. Density ρ 0056 kgm³ and pressure P 3000 Pa.
When the air is further cooled the water vapor will condense to form liquid water dew. 15 Using the numerical values of the ISA constants equation 15 may be. As the temperature decreases the density increases and as the temperature increases it decreases.
This formula can be used not only for liquids but for all fluids. The heat of vaporization is described as the amount of heat required to convert 1 g of a fluid into a fume without changing the fluids temperature. We can do this as follows using standard density molar mass and vapor pressure values for our two chemicals.
The density of a substance can be explained as the relationship between the mass of the substance and the volume it takes up. However as with almost all materials its density changes with temperature. Mathematically density is defined as mass divided by volume.
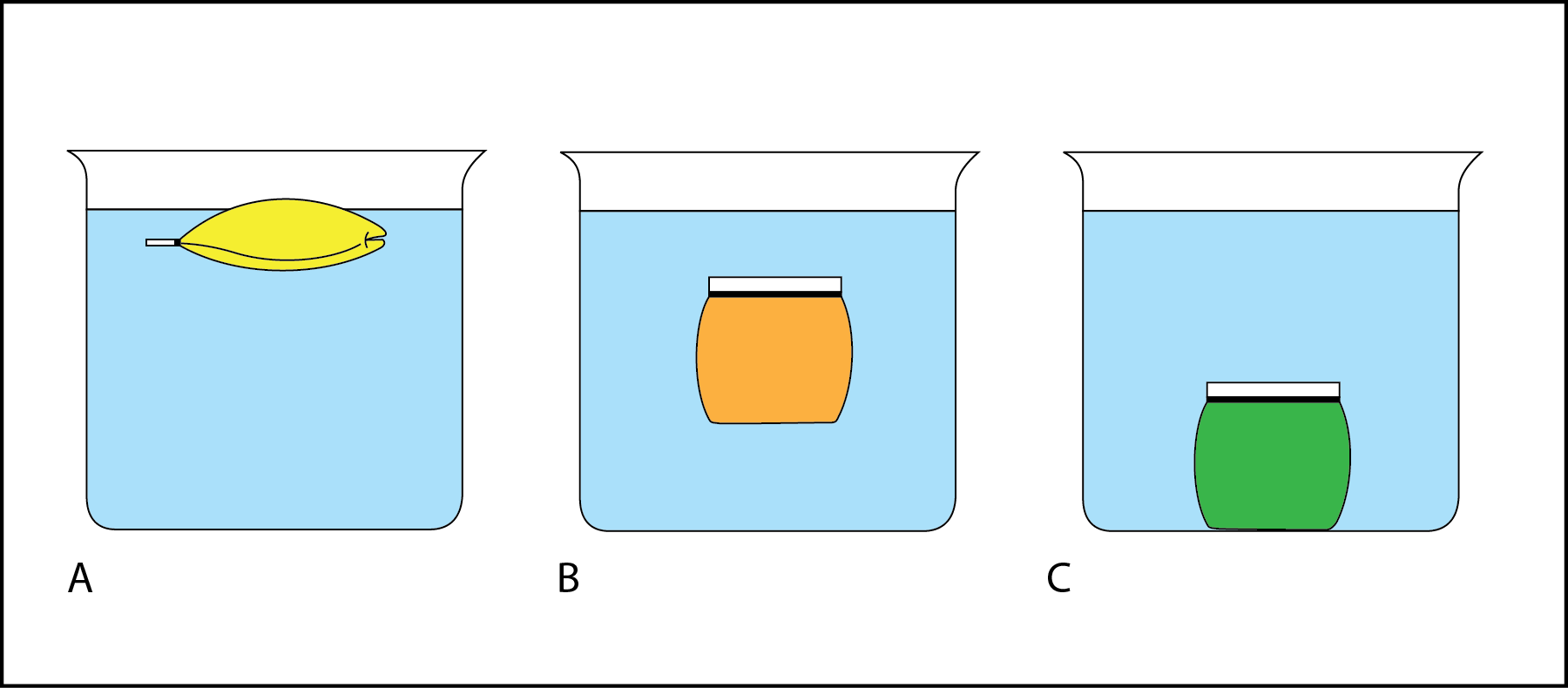
This formula is simply a rearrangement of equations 9 10 and 11. Density volumetric mass density or specific mass is the substances mass per unit of volumeThe symbol most often used for density is ρ the lower case Greek letter rho although the Latin letter D can also be used. When an object is immersed in a liquid it may float or sink in the liquid depending on its density.
DP 24312 α 1762 - α. These figures show that there is an inverse relationship between temperature and density of ethanol. Where ρ is the density m is the mass and V is the volume.
When producing ethanol. If the density of the liquid is more than the density of the object then the object will float on the liquid. Formula of Speed of sound is.
However we have a slight but a super important anomaly when it comes to water. While the general rule is that as temperature goes up the density lowers water behaves differently between 0 C and 4 C. Heat of Vaporization Formula.
The formula for density is the mass of an object divided. For example a result of 1050 means the liquid is 1050 times as dense as water. There are several ways to approximate the dew point.

Liquids Densities Vs Pressure And Temperature Change

Density Of Liquids And Materials 3 Steps With Pictures Instructables
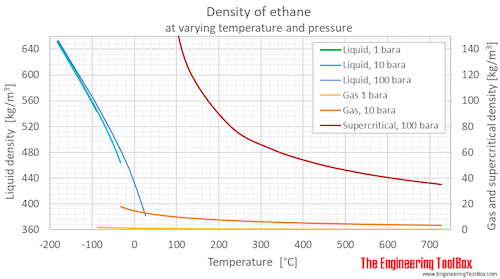
Ethane Density And Specific Weight Vs Temperature And Pressure

14 1 Fluids Density And Pressure University Physics Volume 1
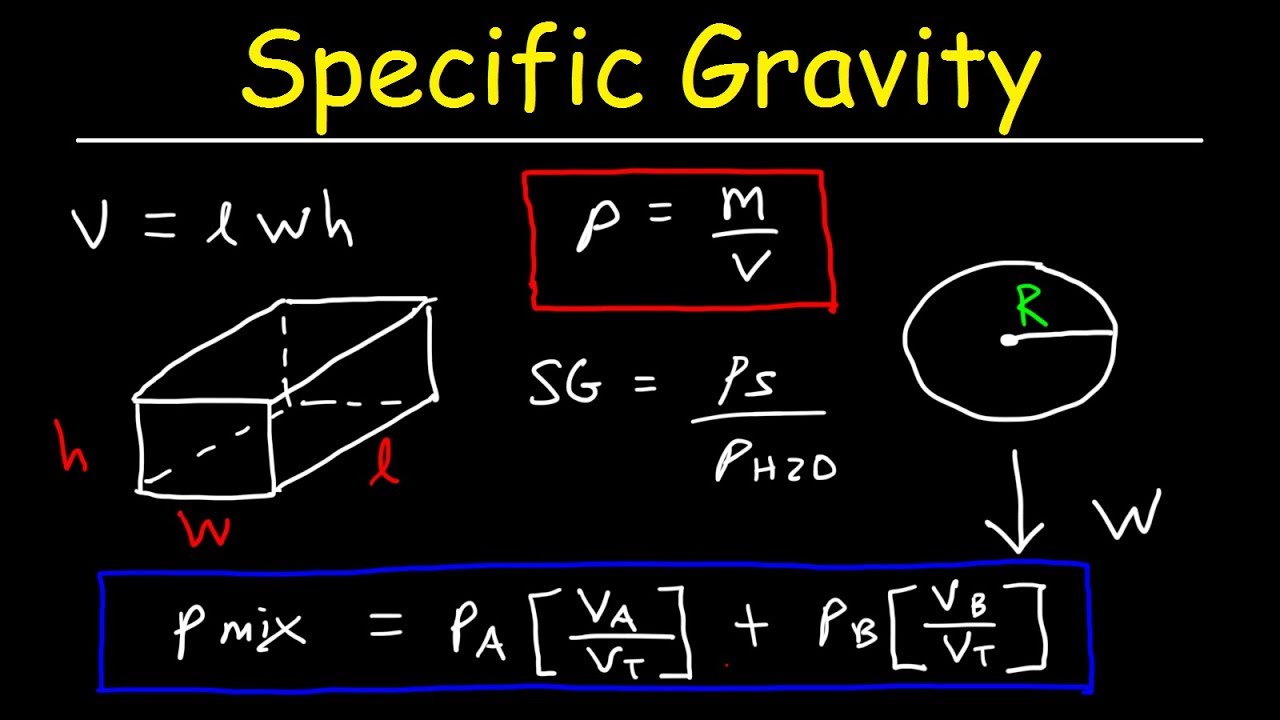
Specific Gravity And Density Of Mixtures Fluids Physics Problems Youtube
Temperature Effects On Density
Density Temperature And Salinity Manoa Hawaii Edu Exploringourfluidearth
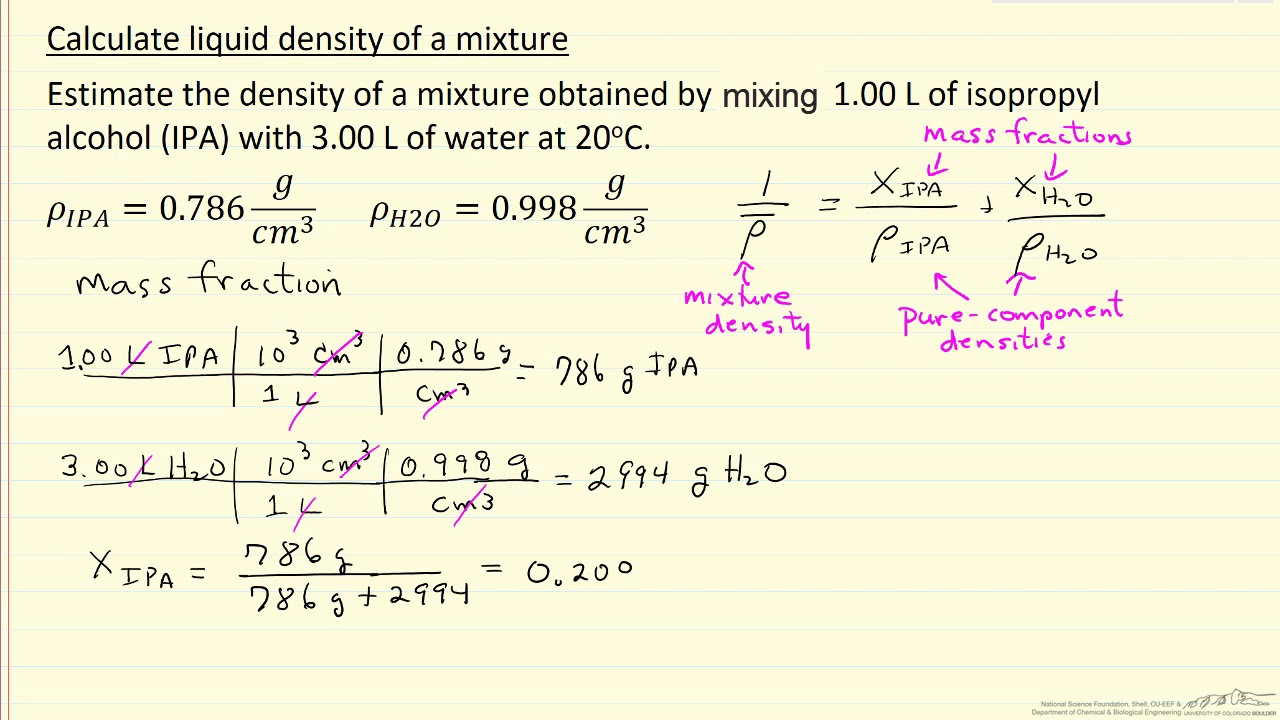
Calculate Liquid Density Of A Mixture Youtube

Weird Science Macroscopic Changes In Liquid Water Volume Manoa Hawaii Edu Exploringourfluidearth

14 1 Fluids Density And Pressure University Physics Volume 1
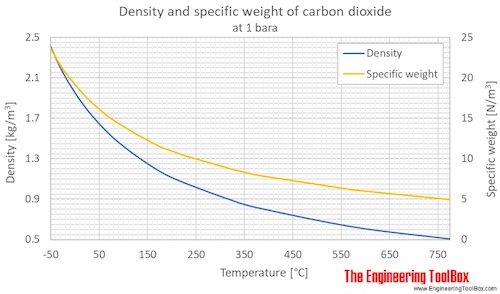
Carbon Dioxide Density And Specific Weight Vs Temperature And Pressure

Density Of Liquids And Materials 3 Steps With Pictures Instructables
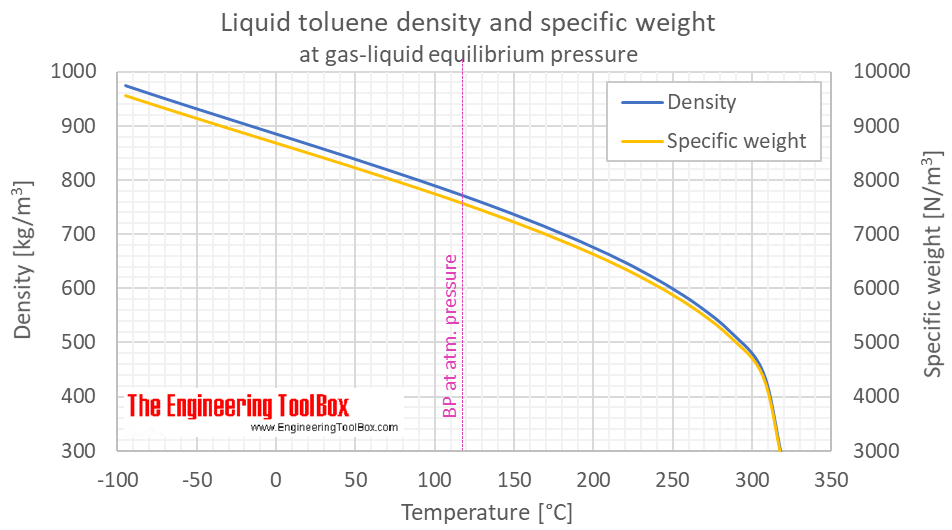
Toluene Density And Specific Weight Vs Teemperature And Pressure
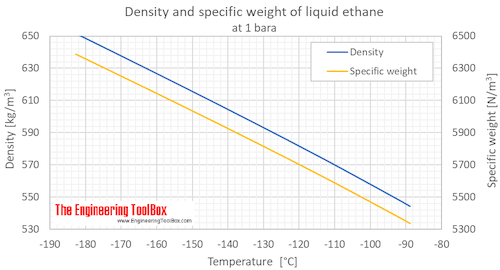
Ethane Density And Specific Weight Vs Temperature And Pressure

Density Of Water Chapter 3 Density Middle School Chemistry
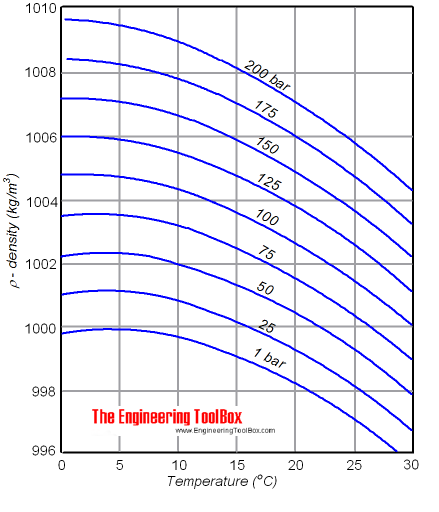
Liquids Densities Vs Pressure And Temperature Change

Density Temperature And Salinity Manoa Hawaii Edu Exploringourfluidearth